Receptors on T and B Cells
Construction of the T-cell receptor
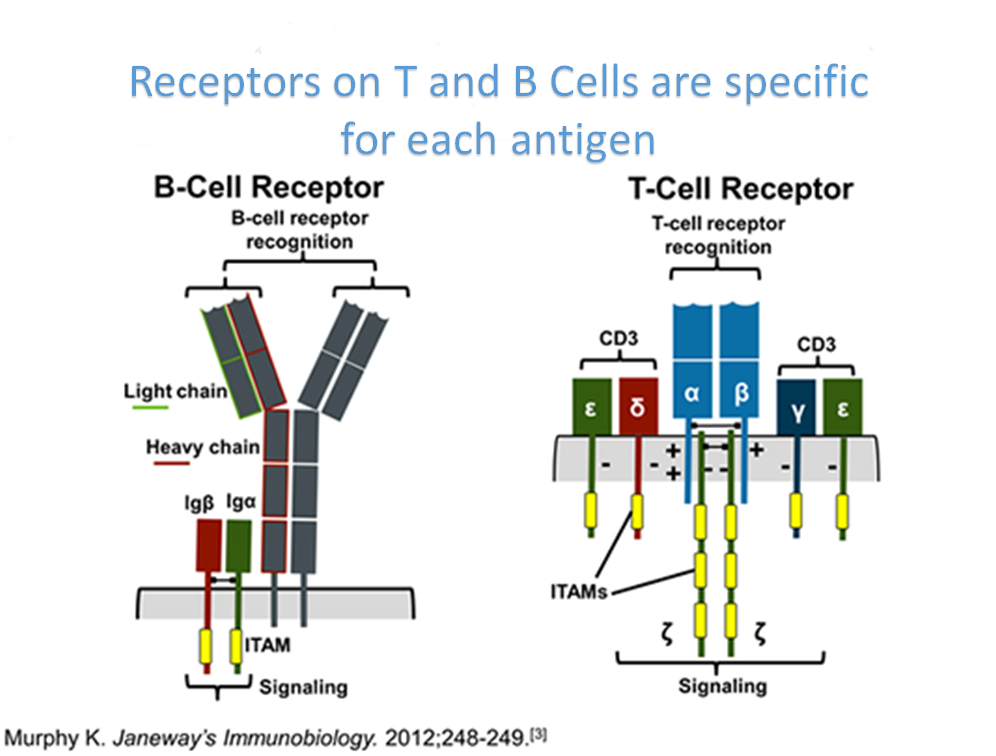
The antibody is not the only protein that recognizes the antigen. The antigen-specific receptor of T lymphocytes does the same. It is simply called the T-cell receptor and is abbreviated as the T-cell receptor TCR. We can define the antigen as a compound capable of eliciting the formation of a specific antibody or T cell receptor.
Unlike the antibody, the T cell receptor is never released from the cell as a free molecule. At all stages of the immune response, it remains anchored in the cell membrane and functions there. Probably because it is not a biologically active ingredient in the serum, the T-cell receptor does not enjoy widespread popularity: non-specialists know nothing about it and students do not like it.
There are two types of T-cell receptors – TCR1 and TCR2. The vast majority of T lymphocytes (over 90%) carry TCR2. Few T-lymphocytes with TCR1 are located beneath the mucous membranes and skin and probably participate in the local protection of the body surfaces. These cells are still poorly understood and appear to play a secondary role. In many respects, they are different from the “conventional” TCR2-bearing T lymphocytes. In the future, when we talk about T lymphocytes, we will only refer to those with TCR2. In the individual cases, when it comes to the minority of T cells with TCR1, this will be explicitly mentioned.
The T-cell receptor is a heterodimer – it consists of two different chains. The two chains that build TCR2 are referred to as alpha and beta, and those that build TCR1 are gamma and delta. There is a disulfide bond between the two chains at each receptor (alpha and beta for TCR2 and gamma and delta for TCR1) that holds them together. The N-terminus of each strand protrudes beyond the cell and the C-terminus is immersed in the cytoplasm. Like most surface proteins, TCR is glycosylated but its sugar moiety is not interesting and we will not dwell on it.
The extracellular portion of each strand consists of two domains supported by one intraschain disulfide bond. In structure, they are somewhat reminiscent of immunoglobulin chain domains. The domain closer to the cell membrane is constant. The other (N-terminal) domain, which is easily accessible from the external environment, is variable – it has different structure according to which antigen recognizes a given T-lymphocyte. The two variable domains of each receptor form its antigen-binding center. Within the variable domain, there are (as with immunoglobulins) hypervariable regions, and it is these that make recognition.
Comparing T-cell receptors to immunoglobulins, we see that both proteins consist of two types of chains that combine their variable parts to form an antigen-binding center. However, the T-cell receptor is monovalent and quite small – corresponds to an immunoglobulin Fab fragment. We know that the vast majority of immunoglobulins have their adapter functions. It is logical that the T-cell receptor, which is not secreted outside the cell and is not loaded with such functions, has a small constant part.
The structural similarity between the immunoglobulins and the T-cell receptor reflects evolutionary homology. Their genes are derived from a common ancestor gene through duplication and divergence. Together with other related genes, they unite into the so-called immunoglobulin superfamily. We’ll meet some more of its members later.
Next to the T cell receptor, a complex of several polypeptide chains, referred to as CD3, is located in the membrane. When the T-cell receptor binds an antigen, CD3 signals this event to the interior of the cell. Because of this tight functional relationship, CD3 is often described as part of the T cell receptor itself. But since CD3 is not involved in antigen recognition and is uniform in all T cells, here by “T cell receptor” we mean only TCR2 or (in rare cases) TCR1.
We will further see that other surface proteins in the immune system are labeled with CD and number. They are initially identified by monoclonal antibodies that recognize them. It is gradually found that the same surface protein is recognized by a whole cluster of monoclonal antibodies. Such a protein is labeled CD and assigned a number associated with the timeline of its detection. Although CD is one of the most important abbreviations in immunology, it does not have a single interpretation and can be referred to as “cluster designation”, “cluster of differentiation” or “cluster determinant” (group determinant) . The name “cluster of differentiation” comes from the fact that CD antigens appear on the cell at a certain stage of its differentiation. In Bulgarian, we can call them “differentiation antigens” (Sarafyan, 2002). CD proteins are also called surface markers because we recognize morphologically similar groups of cells. For example, T lymphocytes may be distinguished from B lymphocytes in that they express CD3.
Main Tissue Compatibility Complex (ICC)
The function of the T-cell receptor is to recognize and bind the antigen. However, this process is not as simple and straightforward as the antigen-antibody reaction. Early cellular immunity researchers were stunned by the fact that T lymphocytes, which are apparently antigen-specific, do not interact with the antigen in vitro. It took years of experimentation to clarify what the T-lymphocyte “sees”. Before returning to this question, we need to get acquainted with another group of cell surface proteins – the molecules of the so-called major tissue compatibility complex.
At the beginning of the twentieth century, oncologists made attempts to transfer tumors. They take cancer cells from one mouse, inject them into another, and monitor if it will also develop a tumor. It turns out that the transfer is successful if the two mice are from the same inbred line. If they are of different lines, in some cases the tumor is intercepted and in others it is not. Whether a mouse line is susceptible or resistant to a particular tumor depends on several genes. Some scientists then suggest that these genes determine the body’s susceptibility to certain types of cancer. Others believe that the genes in question regulate the immune response and that the immune system of resistant mice successfully responds to tumor-specific antigens. However, the question arises why the immune system of susceptible mice, which do not otherwise suffer from immune deficiency, fails. In 1933, the geneticist Haldane offered an answer: tumor rejection is indeed an immune phenomenon, but the immune response is not against tumor-specific antigens, but against normal tissue proteins specific to one or another line of mice. And it really turns out that one line of mice rejects tumors from another if and only when it rejects grafted normal tissues from the same line. The genes that depend on accepting or rejecting the graft are called tissue compatibility genes and the encoded proteins are tissue compatibility antigens.
In the following years, tissue compatibility is being extensively investigated for the possibility of organ transplantation in humans. Tissue compatibility genes are mapped to different locations in different chromosomes, but the most important ones are brought together in a complex locus. These most important genes, as well as the antigens encoded by them, are united under the name major tissue compatibility complex, Soc. Major histocompatibility complex. MHC antigens are membrane glycoproteins, fold.
It can be seen that the MHC genes (and their products) are grouped into
three classes. In fact, only Class I and Class II encode membrane antigens of
tissue compatibility. Genes of so-called class III encode complement components
that are soluble and have nothing to do with tissue compatibility. The only
reason for these serum proteins to be assigned to the MHC is that their genes
are between the class I and class II genes.
Now
we will take a closer look at grades I and II and compare them. Both classes
belong to the immunoglobulin superfamily. Class I antigens possess all nuclear
cells with one important exception – the placental trophoblast. Class II
antigens are more restricted in expression: on some cells of the immune system,
most notably mononuclear phagocytes and B-lymphocytes.
Both
Class I and Class II are heterodimers, i.e. complexes of two different chains.
Class I antigens consist of an alpha chain comprising three extracellular
domains and a non-covalently linked smaller chain than one domain called
beta2-microglobulin. The alpha chain is anchored to the membrane and is encoded
by class I genes. The beta2-microglobulin is not anchored to the membrane. It
is needed in order to support the alpha chain and make it conform. In fact,
beta2-microglobulin is only an honorary member of the MHC because it is encoded
by a single gene with one major allele that is not even in the MHC locus but
elsewhere in the genome (in humans in the 15th chromosome).
Of
the three extracellular domains of the alpha chain, the closest to the membrane
contacts the beta2-microglobulin and the other two are convenient for access
from the external environment. Spatial folding between these two domains forms
a characteristic groove. In trying to purify class I antigens, researchers were
under the impression that some peptides, which had nothing to do with class I, stubbornly
contaminate the preparation. The alpha chain groove has been shown to be able
to accommodate a peptide 8-10 amino acids long and bind to it quite tightly,
albeit non-covalently. In fact, class I proteins occupy their proper spatial
structure only after they have acquired such a peptide and lose it if the
peptide is detached. Normally, the alpha chain is complete with a peptide
before it is brought to the cell surface.
Class
II antigens consist of two chains labeled with alpha and beta. The above table
does not indicate that each of the “loci” for class II human HLA-DP,
DQ and DR actually includes two loci for the alpha and beta chains,
respectively. Both chains are anchored in the membrane and have two
extracellular domains. The two farther from the cell surface domains of the two
chains contact each other and form a groove, analogous to the two outermost
domains of class I MHC. A peptide is also located in this groove. It is
slightly longer than in class I MHC – it consists of 16-20 amino acids. As with
class I MHCs, the peptide is required for the proper conformation of class II
MHCs and joins them even before they reach the cell membrane.
T-lymphocyte epitopes and their recognition
While the antigenic determinants (epitopes) recognized by B-lymphocytes have a different chemical nature, T-lymphocytes recognize exclusively protein antigens.
As mentioned above, early researchers have difficulty studying the interaction of T lymphocytes with the antigen. If purified T-lymphocytes specific for a particular antigen are added to the antigen, they will not bind it. In other words, T-lymphocytes, unlike B-lymphocytes, do not recognize the native antigen.
It turns out that in order to see the T-lymphocyte antigen, it must be shown to them by other cells, which we call antigen-presenting. They have on their surface the antigen, but not native, but processed through incomplete proteolysis, because that’s how T lymphocytes perceive it.
However, this is not the whole story. Antigen-presenting agents may only be cells that express MHC. Moreover, in order to recognize the T-lymphocyte antigen, the antigen-presenting cell must have the same MHC as the T-lymphocyte itself. This phenomenon is called MHC restriction (from Latin restrictio). It is explained by the assumption that T lymphocytes can for some reason recognize the antigen only if they simultaneously recognize their own MHC molecules.
Ultimately, the recognition of antigen + MHC by T lymphocytes is elucidated. We will not dwell on the numerous attempts to achieve this, but we will come to the conclusions directly. Antigens for T lymphocytes are peptides that are found in the groove of MHC molecules. The cell that has brought on the surface MHC with the peptide becomes an antigen-presenting cell for T lymphocytes. The T-cell receptor recognizes its own MHC antigen and its associated peptide antigen as a whole.
Compared to B-lymphocyte epitopes, T-lymphocyte epitopes are fairly
uniform. They are all protein in nature and sequential. This is necessitated by
the need to fit into the groove of a MHC suitable for peptides of a certain
length. In the following sections, when looking at the function of T
lymphocytes, we will explain why it is advantageous for them to recognize their
antigens in such a complex way.
The
mechanism described is valid for the vast majority of T lymphocytes that have
TCR2. Antigenic recognition in T-lymphocytes with TCR1 is still being
investigated, but at least some of them do not appear to be subject to MHC
restriction and may recognize native antigen as B-lymphocytes.
